
From Stardust to Lifeblood: The Epic Journey of Iron
Share
Forged in the Stars: The Cosmic Origin of Iron
The tale of iron begins in the hearts of massive stars. In the early universe, there was no iron at all – the Big Bang produced only the lightest elements (mostly hydrogen and helium). Heavier elements like iron had to be cooked up in stars over millions or billions of years. Here's how it works: as a star ages, it acts like a giant furnace fusing lighter elements into heavier ones. Small stars (like our Sun) mainly fuse hydrogen into helium. But massive stars – those with more than about ten times the Sun’s mass – burn much hotter and can fuse elements in stages, creating carbon, oxygen, silicon, and finally iron in their core. By the time a star’s core turns to iron, it has reached an endgame. Iron is special – fusing iron doesn’t release energy, it actually consumes energy. So when a star’s core fills up with iron, the star can no longer hold itself up against gravity. In a spectacular finale, the core collapses and then explodes outward in a supernova.
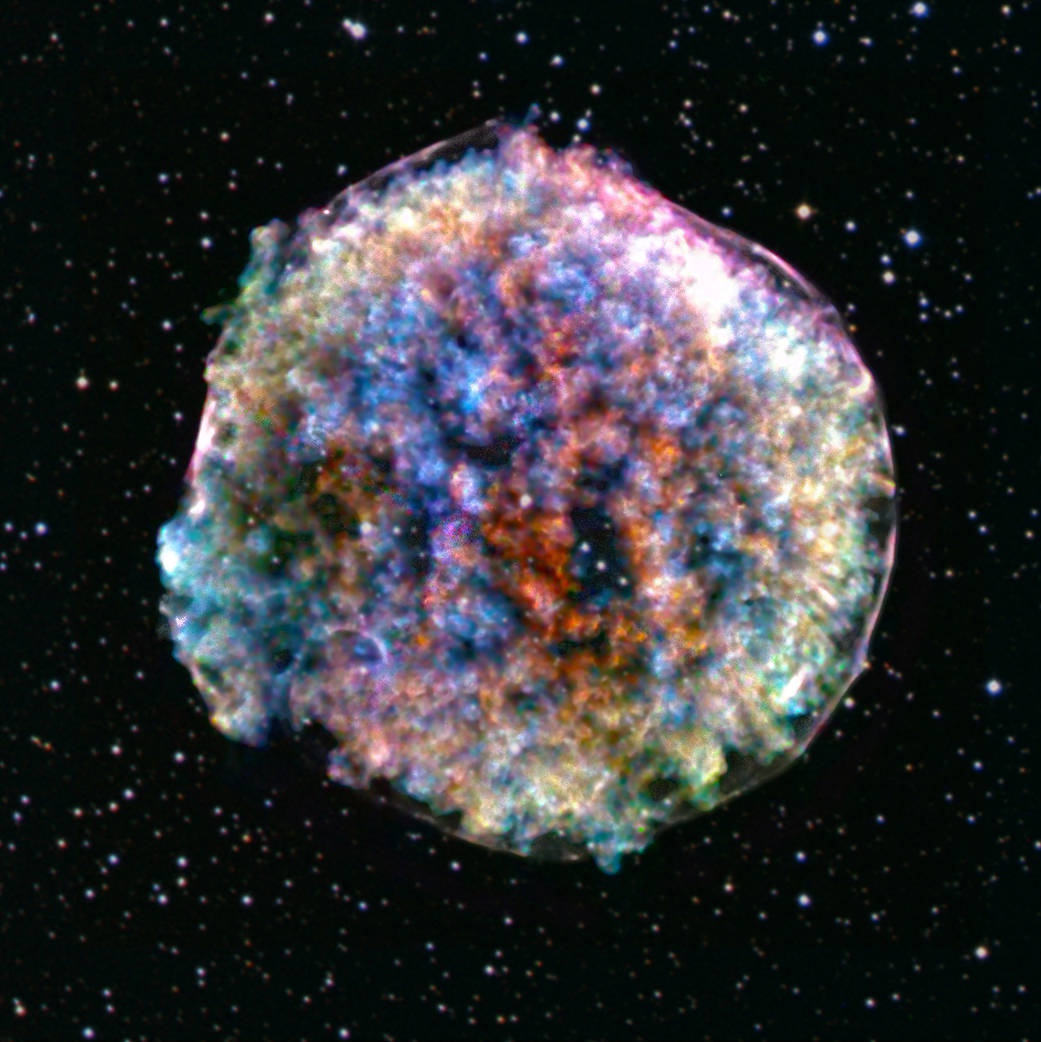
Tycho’s Supernova Remnant: The colorful debris cloud from a star that exploded in 1572. Such supernovae scatter heavy elements like iron into space. Image via NASA.
Supernova explosions are extreme cosmic events – for a brief time a single exploding star can outshine an entire galaxy. More importantly for our story, supernovae scatter elements that the star forged, blasting them into interstellar space. The iron that was locked in the star’s core is violently expelled, mingling with gas and dust in the galaxy. Over eons, this debris from supernovae enriches vast cosmic clouds that will form new stars and planets. In a very real sense, supernovae are galactic recyclers: they take the stellar “ash” (elements like iron) and spread it around to become raw material for the next generation of worlds.
It might sound poetic, but it’s also scientific fact – “Supernovae also provide the iron in your blood,” as a NASA educational page explains. Astrophysicists have calculated that a large fraction of the iron on Earth (and in our bodies) was produced by supernova explosions. In particular, one type of stellar blast, the Type Ia supernova, is known as an efficient iron factory – these explosions produce massive amounts of iron as they tear white dwarf stars apart. Without such cosmic blasts, elements like iron would remain trapped in dead stars instead of being available to form planets or people. We owe our existence to these stellar alchemies.
From Stardust to Earth: Iron’s Planetary Arrival
After a supernova disperses iron and other elements into space, what next? The story pauses for a while in the tenuous interstellar clouds – giant nebulae of gas and dust. Over time, gravity causes parts of these clouds to collapse and form new stars and planetary systems. Our own Solar System was born this way about 4.6 billion years ago, in a cloud already enriched by earlier stars. Thus, the Sun and Earth inherited a portion of those heavy elements forged long ago. Scientists can see this in the Sun’s composition: unlike the very first stars (which had almost no heavy elements), our Sun contains about 2% heavier elements – including oxygen, carbon and iron – that came from previous generations of stars.
When Earth formed out of that solar nebula, it ended up loaded with iron. In fact, iron is one of the most abundant elements on Earth by mass. Most of it sank to the planet’s core during Earth’s formation, which is why Earth’s core is thought to be a ball of iron and nickel. But plenty of iron remained in the crust and mantle – available to become part of rocks, water, and eventually living organisms. Some iron even arrived later via meteorites (many meteorites are rich in iron-nickel metal). It’s awe-inspiring to think that chunks of iron meteorite that ancient humans crafted into tools or jewelry literally fell from the sky – stardust delivered to our doorstep.
So next time you hold a piece of iron – whether it’s a cast iron pan or a steel tool – remember you’re holding cosmic metal. The iron in a simple nail or in your blood hemoglobin originated in stellar furnaces and supernovae billions of years ago. As astrophysicist Carl Sagan famously said, “We are made of star stuff.” Here, star stuff isn’t just a pretty phrase – it’s the iron truth!
Iron: The Life-Giving Metal in Our Blood
All that cosmic journey is fascinating, but how did iron become so crucial to life? It turns out that on early Earth, primitive life forms found iron to be a very handy element. Iron’s chemistry is perfect for shuttling electrons and oxygen around – tasks vital for metabolism. In fact, many ancient enzymes incorporated iron, and some bacteria even breathe iron the way we breathe oxygen! Over billions of years, life evolved to make extensive use of iron’s talents. By the time animals appeared with blood and circulatory systems, iron took on its superstar role: carrying oxygen in the blood.
If you’ve heard of hemoglobin, you know where this is going. Hemoglobin is the iron-containing protein in our red blood cells that gives blood its red color and, more importantly, binds oxygen. Iron is the reason hemoglobin can pick up oxygen in the lungs and release it to tissues throughout the body. In fact, iron is so essential to blood that about 70% of the iron in your body is found in your red blood cells (in hemoglobin) and in muscle cells (in a similar oxygen-binding protein called myoglobin). Every time your heart pumps, billions of iron atoms in hemoglobin grab oxygen molecules and help deliver them to your brain, muscles, and organs. Without enough iron, your blood literally can’t carry sufficient oxygen – which is why iron deficiency makes people feel tired and weak.
Iron doesn’t just live in blood; it’s a key ingredient in many enzymes and proteins that keep the body running. For example, iron is a component of myoglobin in muscle (which stores oxygen for quick use) and is found in enzymes that help our cells produce energy, synthesize DNA, and protect against free radicals. Iron is even needed for immune function – your white blood cells use iron-dependent enzymes to kill bacteria, and your thyroid gland needs iron to make hormones that regulate metabolism. In short, iron is like a versatile multitool: one element supporting dozens of vital processes. It’s no exaggeration to say that iron means life – without it, human biology as we know it wouldn’t work.
Consider this: an average adult only has about 3 to 4 grams of iron in their entire body (roughly the mass of a small nail), yet that tiny amount makes all the difference. Most of that iron is constantly recycled – when red blood cells get old, our bodies salvage the iron and use it again to form new blood cells. The body is pretty iron-thrifty because iron is hard to obtain and precious. Unlike some nutrients, the human body cannot make iron or even excrete much of it; we have to get the iron we need from our environment (food or supplements) and hold on to it tightly. This brings us to a crucial part of iron’s journey: how it goes from soil to our blood through the food chain.
When Iron Runs Low: Deficiency and Anemia
Because iron is so critical, it’s no wonder that iron deficiency is the most common nutrient deficiency in the world. If a person’s diet doesn’t provide enough iron (or their body can’t absorb it well), eventually their iron stores drop and hemoglobin production slows. The result is iron-deficiency anemia – a condition where blood lacks enough healthy red cells to carry adequate oxygen. Anemia due to iron deficiency can leave one feeling constantly fatigued, weak, dizzy, and short of breath. It can also affect concentration, mood, and even body temperature regulation. In children, iron deficiency can impair cognitive and physical development.
Iron deficiency anemia isn’t rare at all – it’s a global public health issue. According to the World Health Organization, roughly 30% of women of reproductive age worldwide are anemic (mostly due to iron deficiency). That’s nearly 1 in 3 women. In children, the rates are also alarmingly high (around 40% of young children worldwide are anemic). Even in developed countries like the United States, iron deficiency affects many people: for instance, about 10–15% of U.S. women are estimated to be low in iron or anemic. Women are particularly vulnerable because of menstrual iron loss and the demands of pregnancy – during which iron needs roughly double to support the growing fetus and placenta. (It’s no coincidence that prenatal vitamins almost always include iron.)
If you’ve ever felt unusually tired or run-down and discovered your iron was low, you’re not alone. Symptoms of iron deficiency often creep up gradually – tiredness, brain fog, pale skin, brittle nails, restless legs – and people may not realize what’s wrong. Many women chalk it up to stress or busyness, not immediately recognizing “the silent drain” iron deficiency can cause on their health (we discuss this in detail in our Iron Deficiency Anemia guide for women, which explores causes and solutions). The good news is that iron deficiency is usually easy to confirm with a blood test (like checking ferritin or hemoglobin levels) and highly treatable once identified.
From Stars to Supplements: Go Wise Iron’s Smart Approach
Think about it: iron’s journey from star core to human bloodstream is an incredible story of nature’s engineering. At Go Wise, we take inspiration from both cosmic and human biology. Our team isn’t your average supplement formulators – it includes PhD scientists across biology, nutrition, genetics, and astrophysics. Why an astrophysicist on a nutrition team? Because we believe that understanding the big picture (literally as big as the universe!) leads to smarter solutions here on Earth. We appreciate that the iron in our product is the same element forged in supernovae, now being used to fuel your cells. It’s a humbling perspective that drives us to get the science right.
When we set out to create our Go Wise Iron supplement, we wanted to solve the common problems that make people shy away from iron pills. The result is a powder-form iron supplement that is gentle, convenient, and effective. Instead of a heavy tablet that sits in your stomach, our iron comes in on-the-go stick packs of powder. You can pour the powder directly into your mouth – no water needed – and it dissolves quickly with a pleasant taste (no nasty metallic flavor). This means you can take it anytime, anywhere, and absorption starts fast. The formulation is tuned to 50% of the typical daily iron dose per stick, which we found is enough for daily support but easier on the stomach. By spreading iron intake out (and not overloading at once), it’s gentle and less likely to cause discomfort.
We also know that vitamin C enhances iron absorption, so we include vitamin C right in the formula. In fact, each stick not only delivers iron but also a boost of key supportive vitamins: Vitamin C, Vitamin B6, Vitamin B12, and folic acid. These nutrients work in synergy – vitamin C improves iron uptake, and B12/folate are themselves crucial for healthy red blood cell formation (preventing other kinds of anemia). It’s a comprehensive daily vitality pack in one. And because the powder is taken by mouth without water, it bypasses some of the common issues; there’s no capsule sitting in your gut to cause irritation, and no metallic aftertaste lingering on your tongue. The feedback from users has been glowing – more energy, clearer thinking, healthier-looking skin – all from a supplement that’s easy to stick with long-term.
(Interested in the specifics? Check out our Go Wise Iron Powder product page for the details on ingredients and the science behind it.)
The Legacy of Iron: Connecting Cosmos and Health
Iron’s saga – from the cataclysmic death of a star to the thriving life of a human – reminds us that everything is interconnected. The iron that colors your blood red and keeps you alive was born in cosmic violence and then delivered across light-years to become part of Earth, part of you. Every time you cook with an iron skillet or take an iron supplement, you’re engaging with a piece of ancient stardust made flesh. This perspective isn’t just a geeky piece of trivia; it’s a powerful reminder of how special even the “common” things in our lives really are.
At Go Wise, we carry this sense of wonder into our work. Our mission is to bridge cutting-edge science with everyday wellness. Knowing the origins and essential roles of nutrients like iron reinforces why we must cherish them. It’s why our team of experts (from PhD nutritionists to astrophysicists) obsesses over formulating supplements that truly make a difference. We don’t compromise on the science, because when it comes to your health – and your connection to the wider universe – every detail matters.
Bottom line: Iron is far more than a supplement you take or something checked in your lab results. It’s a cosmic gift woven into our biology. Ensuring you have enough iron is one of the smartest ways to honor that gift – keeping your body energized, your mind sharp, and your spirit ready to reach for the stars. So the next time you mix up a healthy meal or tear open a Go Wise Iron stick pack, take a moment to marvel. You’re nourishing yourself with an element that traveled across time and space to be here, now, powering your life. And that is truly a story for the ages, written in the language of science and wonder.
Stay wise, stay healthy, and remember: the iron in your blood has a tale as old as the universe – and its next chapter is the vitality and strength it brings to you.
Learn More
Explore these resources to dive deeper into the fascinating science behind iron:

